SYNTHESISING THE MANUFACTURED OF AMMONIA AND ITS SALTS!
Synthesising the manufacture of ammonia and its salts.
A)AMMONIA AND ITS PROPERTY.
Main uses of ammonia :

A)AMMONIA AND ITS PROPERTY.
Main uses of ammonia :

- Manufacture of fertilizers such as ammonium sulphate,ammonium nitrate,ammonium phosphate and urea.
- Manufacture of nitric acid in Oswald Process.
- Manufacture of synthetic fibre and nylon.
- Liquid ammonium is used as cooling reagent in refrigerators.
- Prevent coagulation of latex.
Physical properties of ammonia:
- colourless gas
- pungent smell
- very soluble in water
- Soluble in water form weak alkali
- channges moist red litmus paper to blue
- Reacts with acids to form ammoinium salts
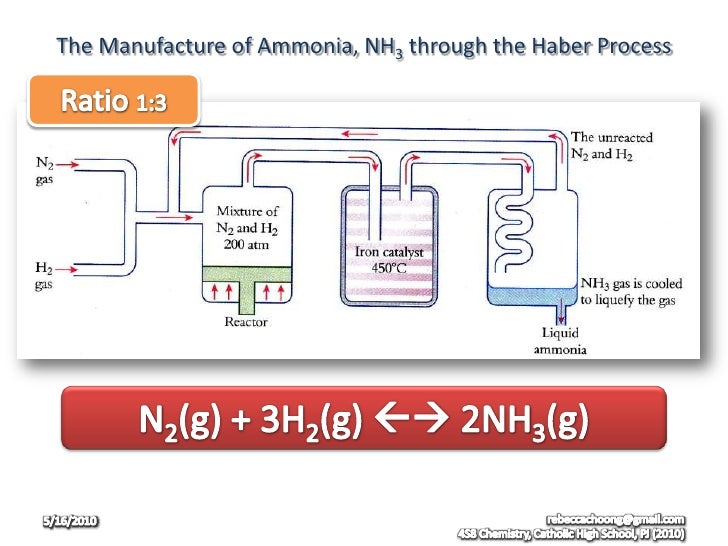
- Manufactured through Haber Process.
- The raw materials are nitrogen gas and hydrogen gas.
- Nitrogen gas was obtained from the distillation of liquid air
- Hydrogen gas was obtained by:
H2O +CH3 →CO2 + H2(mixture of product is known as water gas)
the reaction of steam with natural gas(that contains methane,CH4)
H2O +CH4 → CO2 +4H2
Haber Pricess occurs with:
- Nitrogen gas was mixed with pure and dry hydrogen gas with a ratio of 1:3 in the reactor and was compressed at a pressure of 200 to 500 atmospheres.
- Gas mixture is then passed through a catalyst,iron powder at a tempeture of 450℃-550℃.The ammonia gas was produced.

B)Preparation of ammonium fertilizers.
- Ammonium fertilizers is on of the chemical fertilizers added to soil to replace the element used up by plants.The major nutrient include nitrogen,phosphorus,potasium and calcium.
- Ammonium fertilizers can be prepared from the reaction between ammonia,NH4 and an acid.(neutralisation).ex. Ammonium sulphate,ammonium nitrate,ammonium phosphate.
Comments
Post a Comment